
It’s the genetic equivalent to discovering a new sensory organ in plants.
A team at the University of Missouri Bond Life Sciences Center found a key gene that sniffs out extracellular ATP.
Scientists believe this is a vital way plants respond to dangers, such as insects chewing on its leaves. The journal Science published their research Jan. 17.
“Plants don’t have ears to hear, fingers to feel or eyes to see. They recognize these chemical signals as a way to tell themselves they are being preyed upon or there’s an environmental change that could be possibly detrimental, and they have ways to defend themselves,” said Gary Stacey, a Bond LSC biologist. “We have evidence that extracellular ATP is probably a central signal that controls the ability of plants to respond to a whole variety of stresses.”
ATP (adenosine triphosphate) is the main energy source inside any cell. All food converts to it before being used in a cell, and ATP is necessary to power many of the cell processes that create more energy. Its value as an energy reserve is squandered outside the cell.
Scientists spent years trying to figure out what this compound did while floating outside cell walls. Animal researchers found that answer in the 1990s. They identified the first ATP receptors, now seen to play roles in muscle control, neurotransmission, inflammation and development.
Plant scientists observe similar extracellular ATP responses in plant biochemistry, but until now could not identify the exact receptor for it or what it did.
“We call this new receptor P2K, and it’s unique to plants,” Stacey said. “Even though animals and plants hold some responses in common, they have evolved totally different mechanisms to recognize extracellular ATP.”
Led by Stacey, MU graduate student Jeongmin Choi and postdoc Kiwamu Tanaka screened 50,000 mutant Arabidopsis plants to find ones that didn’t respond to extracellular ATP. Using a protein called aequorin – which causes jellyfish to glow – the two-year process boiled down to whether a plant would produce light when ATP was added. Since aequorin only luminesces when it binds to calcium, those plants without extracellular ATP receptors stayed dark.
“If you add ATP to wild-type plants, calcium concentrations go up and the plants produce more blue light,” Choi said. “We found nine mutant plants with no increase in calcium and, therefore, no increase in light emission.”
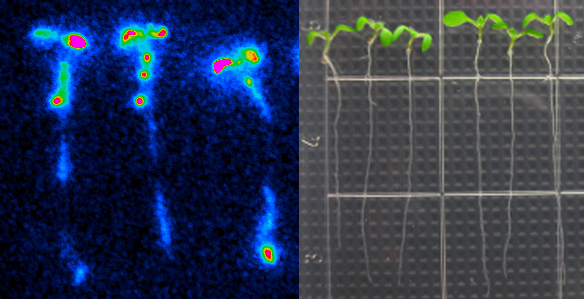
By comparing the genetic sequences of these nine mutants, Stacey’s lab pinpointed the gene to chromosome 5 and labeled it DORN 1, since it doesn’t respond to the nucleotide ATP.
This discovery casts a different light on previous research.
“What we think is happening is that when you wound a plant, ATP comes out in the wound and that ATP triggers gene expression, not the wounding in and of itself,” Stacey said. “We think ATP is central to this kind of wound response and probably plays a role in development, in a lot of other kinds of things.”
Future research will focus on exactly how this receptor works with ATP. Tanaka plans to study its protein structure, how it reacts to pests in lab situations and possible co-receptors that could also play a role in recognizing ATP.
Grants from the U.S. Department of Energy Office of Basic Energy Sciences and the Republic of Korea supported this research.


