Cross-collaborative research team looks to refine delivery of cancer treatments

By Mariah Cox | Bond LSC
“When you want to use a tool to do something in the house, you have to use the right size tool. It does no good to use a large screwdriver to fix the tiny screw on your glasses.”
That’s Donald Burke, Bond Life Sciences Center lead primary investigator, as he begins to explain a project looking to optimize the targeting of cancer cells as part of a large cross-collaborative research team.
And the tools Burke is referring to are aptamers, single-chained synthetic DNA or RNA molecules. Aptamers are tricky molecules. Stemming from the Latin word “aptus,” meaning to fit, and “meros,” meaning part, aptamers must be complementary in size and shape to a certain cell-surface receptor in order to be useful as targeting tools, just like having the right size tool.
Last fall, Burke’s lab was able to identify aptamers as a specialized delivery method that have the potential to carry chemotherapy drugs and imaging agents, or cargo, as little backpacks to diseased sites. An important feature of aptamers is their three-dimensional structures which allow them to bind to target sites with high selectivity. That conceivably means they could deliver a drug to a particular part of the body, like a tumor, and not harm nearby tissues.
Now, the team, comprised of MU experts and researchers who specialize in surgery, radiology, molecular biology and immunology and chemical engineering, is hitting the ground running with a two-year research plan to refine the delivery of cancer treatments. The group was one of 19 innovative research projects across all four UM System campuses to receive a grant from a $20.5 million investment for research and creative works.
“Chemotherapeutics are not very specific and most of them act to block DNA replication or other functions of the cell in both healthy and cancerous cells,” said David Porciani a post-doc who started in the Burke lab in the spring of 2016 after finishing his Ph.D. in molecular biophysics in Italy. “Cells that are dividing are more susceptible to the chemotherapeutic effect, that’s why chemotherapy patients start losing hair.”
In general, therapeutic drugs don’t know to go only to tumors, and they don’t differentiate between cancer cells from healthy cells. The obstacle in only targeting cancer cells is to find specific indicative markers that are unique to tumors. Sometimes researchers have to make do with markers that are overexpressed on tumors cells, but those same markers can also be present in low levels on healthy cells.
The team is working simultaneously towards four major objectives – to identify a comprehensive panel of aptamers that target the majority of tumors, develop molecular tools to enhance the delivery of cargo specifically to cancerous cells, improve imaging for targeted delivery of radiopharmaceuticals, and enhance the efficacy of killing solid tumors through immunotherapy.
Each researcher bringing their unique expertise to the table play an important role in ensuring the project stay on track. Mark Daniels, an associate professor of Molecular Microbiology and Immunology and Surgery from the School of Medicine; Bret Ulery, an assistant professor in the Department of Biomedical, Biological & Chemical Engineering; and Donald Burke, a professor of Molecular Biology and Immunology in the School of Medicine and joint professor of Biochemistry, have been instrumental in the project since its beginning stages.
A collaboration years in the making
“Daniels, Ulery and Burke labs have been collaborating for a number of years, each of us excited about what the other could bring to the collaboration,” said Burke. “[Over the years] we’ve explored several ways of making things move forward, we’ve figured out some productive ways to work together and we’ve identified the key questions that we could pool our respective expertise toward answering,” said Burke.
Daniels’ subgroup of the project has been using flow cytometry, a technique used to detect and measure biochemical and molecular characteristics of tumor cells to see which ones are recognized by a set of different aptamers.
Additionally, Ulery has provided extensive insight into the different ways to package molecules together so that they can move around the body and get to where they need to go.
The grant comes as part of the combined UM system’s and all four campuses mission to supply funding for opportunities that will enhance the ‘well-being for Missouri, the nation and the world through transformative teaching, research, innovation, engagement and inclusion.’
For Burke, the grant couldn’t have come at a better time.
“This team has existed before the announcements were made that there would be these opportunities and so when they announced we said that this was tailor-made for us,” said Burke. “It was just the right time for us. Had they had the same competition three years ago, we weren’t ready for it. Had they had it three years from now hopefully we wouldn’t have needed it anymore and we would’ve already gotten the project to the next level.”
Another key player in the development of this discovery is David Porciani. He has spent three years trying to create ‘smart’ molecules that know exactly where to bind without damaging healthy cells, and he has been working on the project since its beginning.
“There are several ways to target cancer cells. Even on campus, there are different groups that are trying to target cancer cells differently and, in my experience, every strategy has advantages and limitations,” said Porciani. “What I see for this aptamer strategy is that it can provide new molecules that can bind to receptors, and it can also identify new tumor biomarkers.”
Porciani’s portion of the project includes using the advanced microscopy capabilities of the MU Advanced Light Microscopy Core to visualize the kinds of receptors on the surface of cancer cells. This information can be very telling in identifying receptors that are specific only to cancerous cells.
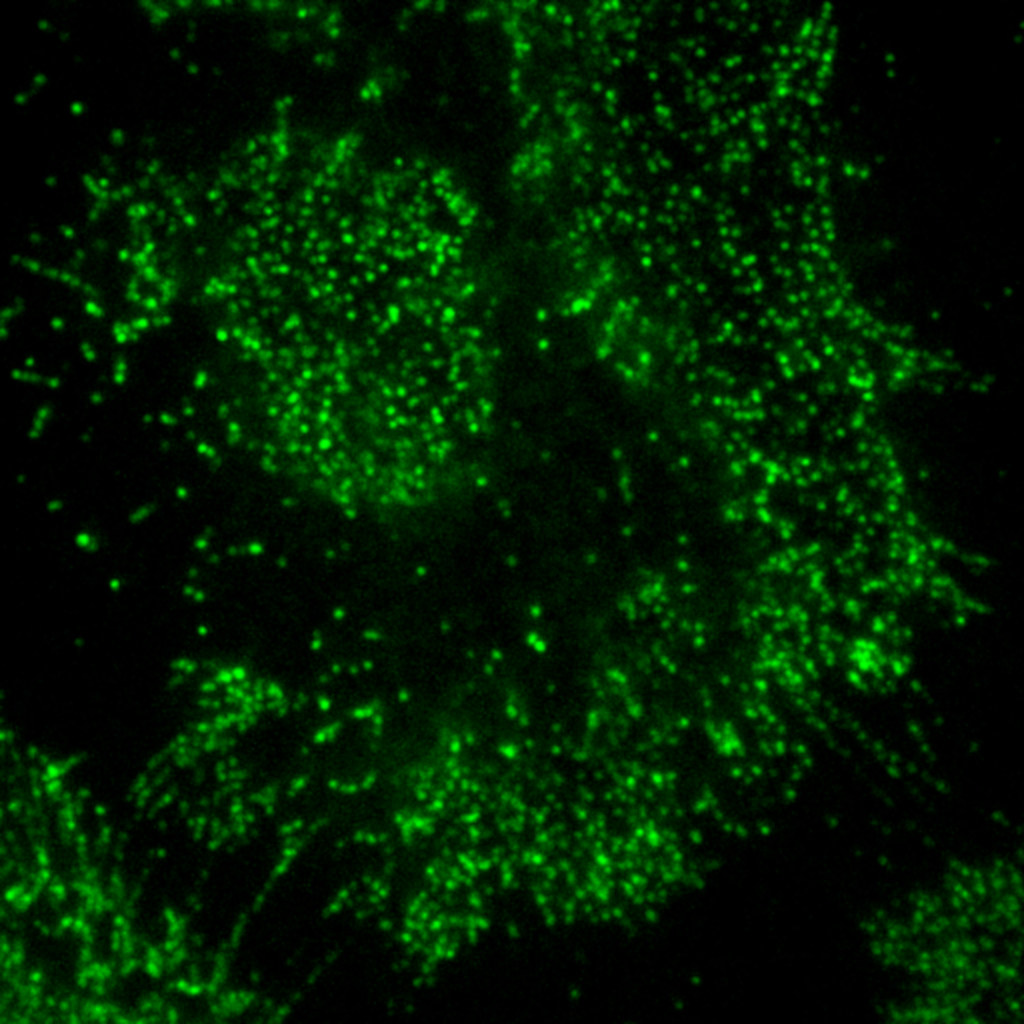
What the future holds
The project now starts down this ambitious road. In the first year, the collaborators are working on discovering their panels of aptamers, developing lung cancer-specific T cells which provide artificial cell receptors for the use of immunotherapy, and beginning testing of the specificity of aptamer-cargo constructs in samples acquired from the American Tissue Cancer Collection. By year two the group hopes to begin testing in biopsy tissues acquired from MU hospital patients acquired under informed consent and in lab mice.
“After we test a specific drug in cell culture samples and see an anti-cancer effect, killing only the cancer cells and leaving the healthy cells, we expect to see the same effect in biopsy tissue samples,” said Porciani. “Having a reduction of the tumor mass and not having side-effects is what we hope to see in biopsy tissues and mice.”
After the end of year two, the team is looking forward to expanding this research to other types of tumors to see if their tumor-targeting method can apply in different cancer types.
“Our team has been trying to piece this together for a long time and it’s been surviving on goodwill up to this point. This is the most substantial funding we’ve had for it to date and we’re really excited that the University of Missouri has chosen to support us on this,” said Burke. “We’re very hopeful that we can use it as the starting point for building a much larger enterprise centered around tumor targeting in general, whether it’s for therapeutics, diagnostics or other purposes.”
Donald Burke is a primary investigator in the Bond Life Science Center and is a professor of Molecular Microbiology & Immunology in the School of Medicine and a joint professor of Biochemistry and Bioengineering. David Porciani is a post-doctoral researcher in the Burke lab. Mark Daniels is an associate professor of Molecular Microbiology and Immunology and Surgery in the School of Medicine. Bret Ulery is an assistant professor in the Department of Biomedical, Biological and Chemical Engineering in the College of Engineering. Other key collaborators on the project include Diego Avella Patino and Jusuf Kaifi in the Department of Surgery and Jeff Smith in the Department of Radiology.

