
It’s an understatement to say viruses are small.
But an average virus dwarfs the diminutive variety known as parvoviruses, which are among the most minuscule pathogens known to science.
Tucked inside a protective protein shell, or capsid, parvoviruses contain a single DNA strand of about 5,000 nucleotides. If parvo’s genetic material is like an hour-long stroll around your neighborhood, a bigger virus like herpes is equivalent to walking from St. Louis to Columbia, Missouri.

“I joke that we can do the whole parvovirus genome project in an afternoon, because it’s just taking it downstairs and having it sequenced,” said David Pintel, a Bond Life Sciences Center virologist and Dr. R. Phillip and Diane Acuff endowed professor in medical research at the University of Missouri. “It’s the size of one gene in the mammalian chromosome.”
But that little stretch of DNA still has plenty of tricks up its sleeve.
Pintel has spent nearly 35 years studying parvo and is one of the world’s foremost experts on the virus, but he’s still plumbing the tiny pathogen’s depths.
His lab focuses on unraveling how parvo interferes with a host cell’s lifecycle and understanding the virus’ quirky RNA processing strategies.
“Even though the virus is small, it’s not simple,” David Pintel said. “Otherwise we’d be out of business.”
Over the last two decades, parvo has become an important tool for gene therapy, an experimental technique that fights a disease by inactivating or replacing the genes that cause it. Researchers enlist a kind of parvovirus known as adeno-associated virus as a gene therapy vector, the vehicle that delivers a new gene to a cell’s nucleus. Pintel helped suss out the virus’ basic biology, an important step for developing effective gene therapy.
A varied virus
The name ‘parvo’ comes from the Latin word for ‘small.’ But the virus’ size makes it a resourceful, versatile enemy and a valuable model for understanding viruses and how they interact with hosts.
Parvoviruses fall into five main groups. They infect a broad swath of animal species from mammals such as humans and mice to invertebrates such as insects, crabs and shrimp.
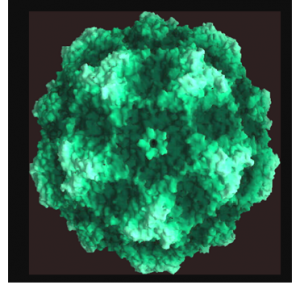
Canine parvovirus, or CPV, is perhaps the best-known type.
It targets the rapidly dividing cells in a dog’s gastrointestinal tract and causes lethargy, vomiting, extreme diarrhea and sometimes death. In humans, Fifth disease, caused by parvovirus B19, is the most common. This relatively innocuous virus usually infects children and causes cold-like symptoms followed by a “slapped cheek” rash. There is no vaccine for Fifth disease, but infections typically resolve without intervention.
Reading the transcript
Pintel surveyed the whole parvo family to understand its idiosyncrasies.
To study bocavirus – a kind of parvo recently linked to a human disease – Pintel looked closely at the dog version, minute virus of canines (MVC). MCV serves as a good model for the human disease-causing virus. While examining MVC, he noticed an unexpected signal in the center of the viral genome. The signal terminates RNA encoding proteins for the virus’ shell, a vital part of the pathogen.
Finding such a misplaced signal in the middle of a stretch of RNA is like coming across a paragraph break in the middle of a sentence.
Pintel knew the virus bypassed this stop sign somehow, because the blueprint for the viral capsid lies further down the genome.
To overcome this stop sign, this particular parvovirus makes a protein found in no other virus. The protein performs double-duty for the virus: It suppresses the internal termination signal and splices together two introns, or segments of RNA that do not directly code information but whose removal is necessary for protein production. Splicing the introns together ensures that the gene responsible for producing the viral capsid is interpreted correctly.
Viruses and hosts: a game of cat and mouse
The conflict between a virus and a host is a constantly escalating battle of assault and deception.
Viruses need a host cell’s infrastructure to replicate, but have to fool or outmaneuver its defenses.
Pintel discovered one example of this trickery in mice, where parvo triggers a cellular onslaught known as the DNA damage response, or DDR. This type of parvo co-opts that defense. Normally DDR pauses the cell cycle to keep damaged DNA from being passed on to the next generation of cells, but parvo exploits that delay, buying time for the virus to multiply.
“For many, many hours the cells are just held there by the virus while the virus continues to replicate,” Pintel said. “And then that cell never survives; the virus kills the cell. It’s that holding of the cell cycle — which is part of the DNA damage response — that the virus hijacks to hold the cell cycle. It’s really cool.”
Parvo’s small size makes it especially beholden to their hosts. But that can make them particularly revelatory for researchers.
“It’s a twofold thing,” Pintel said, “Because it’s a virus that’s dependent on the cell, when you learn how the virus is doing these things, you learn how the cell does those basic processes. If we’re looking at a viral-cell interaction, yes, we’re looking at it from a viral point of view, but on the other hand we’re trying to understand the basic cellular process.”
Uncovering such nuanced interactions is a painstaking, laborious process that often goes unheralded by mass media. But those fundamental discoveries provide the building blocks upon which other researchers depend, said Femi Fasina, a postdoc in Pintel’s lab.
“When you understand basic biology, people can walk on those advancements. Although we don’t see the impact immediately, such things lead to breakthroughs that will revolutionize a lot of things.”
A small question
Despite his deepening understanding of how parvo works, there remains one debate about the virus that Pintel deems beyond the scope of his research: Are the tiny slivers of DNA that comprise parvoviruses even alive?
“I think that’s a crazy question,” he said. “It’s semantics. The virus is a genome. It goes into a cell, it doesn’t do anything until it’s inside of a cell and then it does stuff.” So whether you write parvoviruses into the book of life depends entirely on how you define the word ‘alive.’
“I put that in the realm of philosophy,” Pintel said, “not the realm of science.”

