By Phillip Sitter | MU Bond Life Sciences Center
For a tiny spore, anthrax holds a lot of danger and promise.
If you found yourself wondering about more than its safety in the lab, we have a answers to a few persistent questions.
What makes anthrax dangerous, and how does it spread? How common are infections of it in nature? If we have antibiotics that already treat it, beyond finding new and better ones why study the organism?
Bovine slayer and bio-weapon
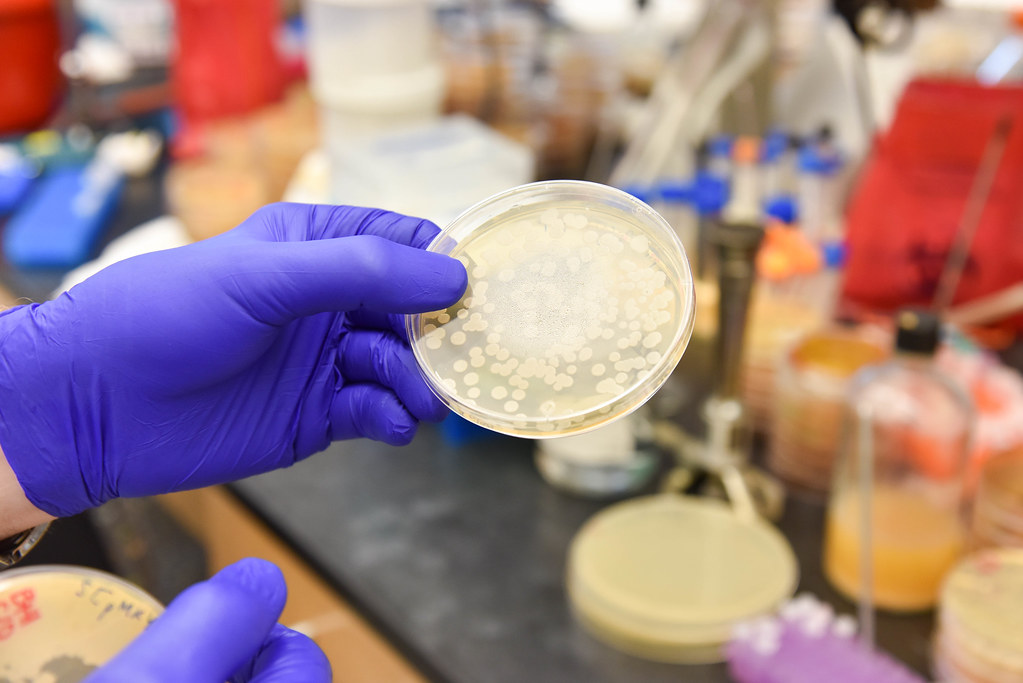
Anthrax bacteria are highly resilient organisms. Like only a few other bacteria in nature, they produce spores with protein-shelled casings that lay dormant in soil for long periods of time, waiting to be taken in by grazing animals like cattle and sheep.
Once they enter the nutrient-rich environment of an animal’s bloodstream — exactly how the bacteria gets there after entering an animal’s mouth is unknown — spores germinate inside the white blood cells that absorb them. As anthrax reproduces rapidly inside its host, it releases toxins that quickly kill the infected animal. That sometimes happens in just a matter of hours according to farmers’ accounts, said George Stewart, Bond Life Sciences Center scientist, medical bacteriologist, McKee Professor of Microbial Pathogenesis and chair of Veterinary Pathobiology at MU.
When infected animals die the bacteria are exposed to oxygen in air that penetrates the decomposing body and new spores escape as the dead host decays. The newly-produced spores are deposited back into the soil, where they wait in a state of suspended animation for as long as it takes to be ingested by another grazing animal, sometimes decades to a hundred years.

Sporadic, natural outbreaks of anthrax can happen almost anywhere in the world except Antarctica, as the spores have been found to exist worldwide, Stewart said. In the United States, outbreaks in cattle and bison usually happen in the Plains and West in states like Colorado and Wyoming — anywhere that cattle or sheep have been raised or their wildlife equivalents graze, lending to the descriptor from Stewart of “anthrax belt” for the states stretching from Texas to the Dakotas.
During the Cold War, the U.S. and the Soviet Union, among others, were attracted to anthrax for their respective biological weapons programs because of the hardiness of the organism and high lethality rates of untreated gastrointestinal and, especially, pulmonary anthrax infections in humans. Today, Americans’ are probably most familiar with anthrax from the mailing attacks of 2001, when letters containing weaponized anthrax spores were delivered to the offices of media outlets and politicians, infecting 22 people and killing five.
Hero in a half-life shell?
Despite their grim reputation, anthrax spores hold a lot of potential for Stewart.
The outermost layer of the protein-shell structure of a spore holds particular interest — how it’s made, what proteins it’s composed of and the function of those proteins. Studying it could not only help find future anthrax vaccines and therapies, but also be used for other applications.
Scientists have coated spores of a close biological relative of anthrax, Bacillus thuringiensis, with plant growth-promoting and anti-insect enzymes and other proteins to treat crops. Its durable structure in the form of the spore’s protein shell attach to these enzymes and remain longer in the environment, making them more effective, Stewart said.
This long biological half-life even presents potential for bio-remediation work — using natural organisms to cleanse the environment of toxins and pollutants. In the case of B. thuringiensis, this might include cleaning up soil from the herbicide atrazine and the notorious pollutant dioxin, a by-product of various industrial processes including incineration, smelting and the production of paper pulp and some herbicides and pesticides.
According to collective findings of the National Institutes of Health and Environmental Protection Agency and others, atrazine is probably not a human carcinogen but can cause genetic damage in animals and is still acutely toxic to people at high enough levels. According to the World Health Organization (WHO), dioxin causes cancer and other ill human ailments.
Pollutants that contaminate soil can eventually leech into groundwater and also enter the food supply through grazing animals. That makes organisms adapted to living in soil like B. thuringiensis perfect candidates as potential carriers of agents used to clean up soil pollutants like atrazine and dioxin.
However, Stewart said that there is too much public stigma to use anthrax for similar applications, “which is too bad because Bacillus anthracis actually works a little better in these applications.”
Rethinking the anthrax image
So is anthrax a dastardly bio-villain or a misunderstood hero?
The truth is neither. Anthrax bacterium is just another living thing.
As a predator of other living things, needing to feed to survive and reproduce, it goes about its life cycle without any conscious agenda. It has no malice, and unlike a wolf, shark or hawk it doesn’t have a brain so it doesn’t even have instinct, only genetic code to mindlessly live by.
How we interact with anthrax is largely dependent upon us humans.
We have the vaccines to prevent it and antibiotics to treat it if it makes us sick. After that, we can harness and modify anthrax’s natural power for ill as a weapon, or maybe to do some good in the environment. So long as we have the technology to study and manipulate organisms like anthrax, the potential for both scenarios will always be there.
Meanwhile, at MU Stewart will continue to study anthrax, searching for its secrets in order to better understand it, to better serve the rest of us.