By Cara Penquite | Bond LSC
Not all tumors are created equal, and potential treatments aren’t universal. When it comes to pancreatic cancer, surgery and radiation often do more harm than good due to its rapid growth and ability to spread to the liver.
Searching for alternative treatment options, Jing Zhou focused her research on immunotherapies. That led Zhou and her team to identify the sequences for over 9,000 cells from pancreatic tumors to out why some therapies don’t measure up.
“My professor said, ‘You have to do a lot of work to try and make sure your sample is ready [and] your experiments are ready. Otherwise, we [will] spend a lot of time, a lot of money, on [research] that doesn’t work,’” said Zhou, a NextGen graduate student who collaborated closely with Trupti Joshi’s bioinformatics lab at the Bond Life Sciences Center for the data analysis.

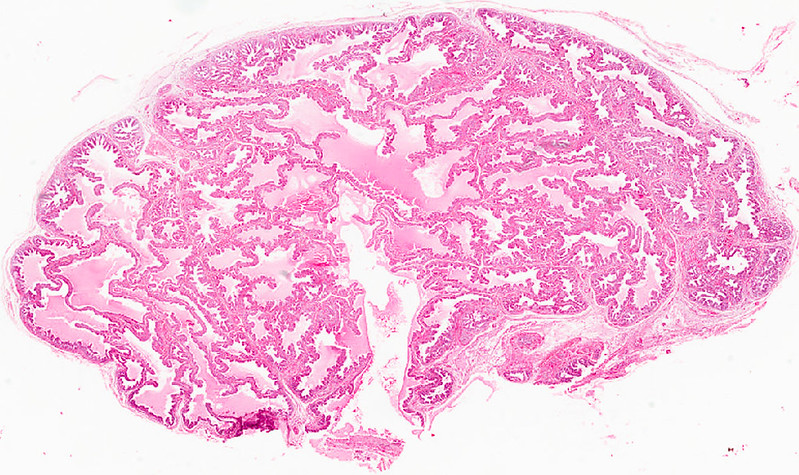
Zhou identified differences between types of pancreatic tumors to understand why certain types of pancreatic cancer respond differently to treatment. Scientists analyzed RNA sequences for two different types of pancreatic tumors. While the project is continually evolving, her most recent findings were published online in the Journal of Translational Oncology.
Zhou focuses on a form of immunotherapy that increases the effectiveness of the immune system. Known as immune checkpoint inhibitors, the treatment turns off protein pathways that act as brakes on the immune system. In cancer patients, turning off the brakes allow immune cells, like T-cells, to attack more cancer cells.
“The cancer cells grow so fast, they expand a lot and that attracts a lot of T-cells. Normally, in a healthy tissue they don’t have so many T-cells, just a few of them to keep the body normal,” Zhou said, “But when you have a strong immune response, like in a tumor, it will have a lot of T-cells in that kind of environment.”
Not all types of pancreatic tumors respond well to immunotherapy.
“This was a very interesting project because what they were doing here is comparing four different types, [but] mainly two different types, of pancreatic cancers,” said Joshi, Bond LSC principal investigator and Assistant Professor at the Department of Health Management and Informatics in the School of Medicine and core faculty in MU’s Data Science and Informatics Institute. “One that is resistant to and one that is sensitive to this anti-PD-1 antibody treatment, what is known commonly as immunotherapy.”

Tumors are composed of many types of immune cells mixed in with cancerous cells, and Joshi’s lab analyzed the composition to understand why two different types of pancreatic cancer react differently to treatment.
Unlike past analyses that relied on analyzing the tumors as a whole, the scientists used a process called single-cell RNA sequencing which allowed them to identify the various types of cells present and quantify the gene expression in individual cells. This quantification allowed the researchers to see which genes were turned on in each cell.
“The single-cell technology is the biggest advancement in sequencing technologies,” said Yuexu Jiang, a postdoc in Joshi’s lab who analyzed the single-cell RNA sequencing for the project.
Joshi suggested the analogy of a fruit smoothie to understand the concept of single-cell RNA sequencing. A fruit smoothie is a mixture of many fruits, and to understand what causes the flavor of the smoothie you must know how much there is of each fruit.
Single-cell RNA sequencing allows the researchers to quantify what types of cells are in the tumor, as well as identify the genetic makeup of the cells in the tumor.
“That really allows you to pinpoint that it is this particular gene expression in this particular type of cell which is really associated with what outcome you’re seeing in terms of these different tumors’ behavior,” Joshi said.
Pancreatic cancer is difficult to treat since surgery and radiation often do more harm than good. With its rapid growth rate and ability to easily spread throughout the body, a pancreatic cancer diagnosis comes with a 50% five year survival rate according to the American Cancer Society.
“I have been involved a lot of times in the surgery. I saw when the surgeon opened the [patient], and when they saw the metastasis, tiny things, on the liver, they just closed the [patient]. They won’t remove the cancer anymore,” Zhou said.
Immunotherapy provides another option for patients that need one.
“Getting a better understanding of the composition and what parts of the immune system are associated or involved in times when you see a cancer responding to immunotherapy […] that is really what this research was targeted towards understanding,” Joshi said.
This research published in the Journal of Translational Oncology November 9, 2021, under the title “Single-cell RNA sequencing to characterize the response of pancreatic cancer to anti-PD-1 immunotherapy.”