Research offers future option to nitrogen to boost crops
By Mariah Cox | Bond LSC
At the age of eight, Beverly Agtuca held lofty aspirations for her future. While many of her classmates wanted to be a veterinarian or a doctor, she dreamed of finding a way to increase plant productivity on her relatives’ farm in the Philippines.
While many children’s career goals change — sometimes more than once — Agtuca held on to her interest in plant science, wrapping up five years of work on her dissertation with a Ph.D. in Plant, Insect and Microbial Sciences last May. Months later, the fruits of her labor made it into the American Phytopathological Society Journal in December and was chosen as the journal’s Editor’s Pick in February.
Agtuca’s research found a potential novel alternative to fertilizing grass species, such as corn — a global staple for human and livestock consumption.
A large-scale problem
Much of the agricultural industry relies on nitrogen fertilizers to support farming. However, when plants can’t absorb nutrients from nitrogen fertilizer quickly enough, soil bacteria convert it to nitrate. It is then flushed out of soils in runoff, polluting groundwater, streams, estuaries and oceans.
“Nitrogen fertilizers are being used worldwide to increase plant productivity. Unfortunately, nitrogen fertilizers have a lot of environmental impacts like pollution and use a lot of energy to make it,” Agtuca said.
Nitrogen runoff from farm fields causes harmful algal blooms — such as red tides, blue-green algae and cyanobacteria — and can have severe impacts on human health, aquatic ecosystems and the economy. In 2018, a red tide event off the coast of Florida killed at least a hundred manatees, a dozen dolphins, thousands of fish and 300 sea turtles to wash ashore in masses. A giant dead zone where the Mississippi River meets the Gulf of Mexico is also a side effect of these algae blooms removing oxygen from the water and rendering it uninhabitable by animals.
While it may seem like we’re only experiencing the effects of nitrogen fertilizer now, it has been around for over a century. More than 175 years ago, scientists in Europe were looking for ways to feed their growing nations and were on the cusp of discovering nitrogen’s role in plant growth. In 1909, German scientist Franz Haber developed a high-temperature, energy-intensive process to synthesize plant-available nitrate from the air, thus creating nitrogen fertilizer.
For over a century, nitrogen fertilizers have helped feed global populations. But its effects are becoming harder to ignore. To keep up with the constant demand for food while considering nitrogen fertilizer impacts, scientists are looking for alternative means to support global agriculture.
Finding an alternative
Recognizing the global impacts of traditional fertilizers, Agtuca set out to learn more about bacteria’s role in promoting plant growth in grass species.
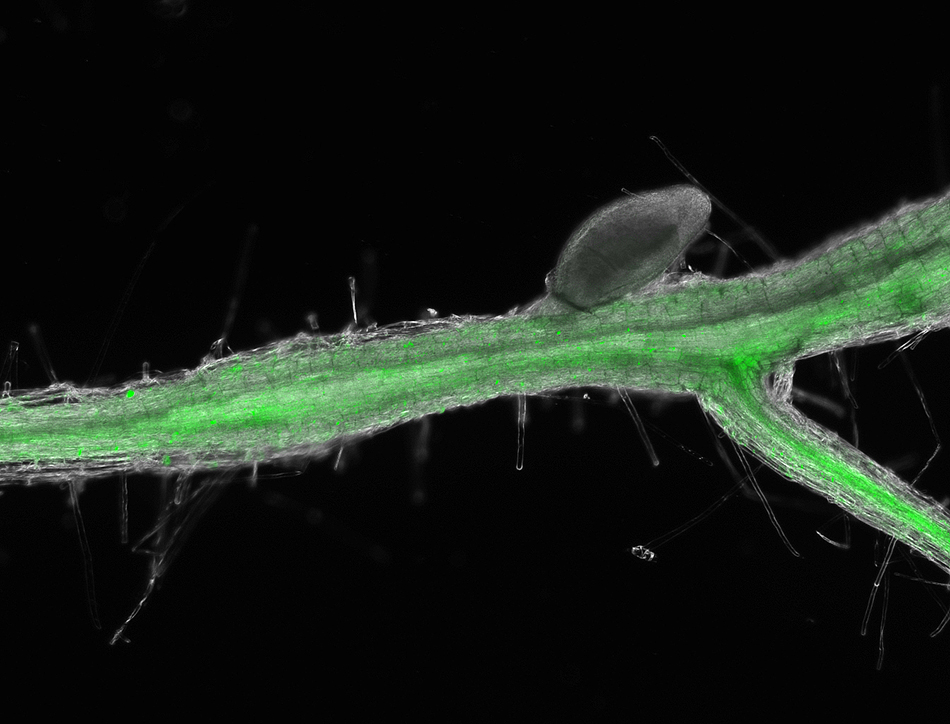
The endophytic bacteria, Hebaspirillum seropedicae, was known to provide hormones to grass species, but not much else was understood about its interaction in plants. To see the interaction between her model species of grass, Setaria, and the bacteria, Agtuca’s collaborator, Sylwia Stopka built a laser ablation electrospray ionization mass spectrometry machine that performed in situ metabolic analysis.
“We found a lot of metabolic pathways that were significant promoters of plant health,” Agtuca said. “Those metabolic pathways were similar to a known nitrogen-fixing bacterium called rhizobium in soybeans, which people previously thought wasn’t similar. And then we found out that it’s more complex than other types of bacteria.”
Agtuca and her colleagues found purine, zeatin and riboflavin pathways in the plant-bacteria interaction — chemical pathways that support plant growth.
“The purine pathway helps to give nitrogen to the plant, whereas zeatin and riboflavin help for plant growth,” Agtuca said. “The bacteria that was found to be beneficial to soybeans formed external nodules on the plant, but this new bacterium does not create nodules. The bacteria live inside the roots intercellularly and only benefit grass species like corn. Corn is an important crop for farming and for our productivity to feed the world.”
While Agtuca’s research won’t be debuting in farm fields anytime soon, it laid the groundwork for other scientists to continue researching new natural fertilizers in hopes of someday replacing chemical fertilizers. The next step is finding a particular molecule to add to the plant, rather than adding bacteria.
After graduation, Agtuca moved to Colorado to teach at Adams State University, a small undergraduate institution in the San Luis Valley. She’s spent her time teaching classes such as microbiology, cell biology, bioinformatics and cell physiology. Although she enjoys teaching, she misses being in the lab.
“It’s great to teach the overall broad concepts to the students and then I can relate it to research, which is fascinating to the students. I try to talk about why we’re learning specific material and why It’s important to our everyday lives,” Agtuca said. “Since I’m freshly graduated, I can express how research works, despite not having research here on campus.”
Her publication ‘In-Situ Metabolomic Analysis of Setaria viridis Roots Colonized by Beneficial Endophytic Bacteria’ was published in December 2019.
Beverly Agtuca reflects on her collaboration with Sylwia Stopka:
Sylwia and I shared the same “Ph.D. experience,” where we were both graduate students and we struggled when experiments don’t work. Together, we both planned how to make the experiment work. We supported and helped each other out. For instance, I was the plant biologist, while Sylwia was the chemist and we both have the same goal in this project. I thank Sylwia because without her we won’t get our findings rapidly and obtained numerous publications including this one.